Southparksteroids.com
Southparksteroids.com
TELEGRAM: @southpark11
TELEGRAM: @southpark11
Email:southpacksteroids@gmail.com
Email:southpacksteroids@gmail.com zz0.2supysnmc9ezz
An official website of the United States government
Here is how you know
NIH National Library of Medicine NCBI
Search PubChem compound Summary Testosterone Propionate
PubChem CID
5995
Structure
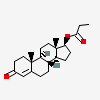 [image]
[image]
 [image]
[image]
Chemical Safety



Laboratory Chemical Safety Summary (LCSS) Datasheet
Molecular Formula
Synonyms
- testosterone propionate
- 57-85-2
- Agovirin
- Androlon
- Androteston
View More…
Molecular Weight
344.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:
2005-03-26 - Modify:
2024-11-09
Description
Testosterone propionate appears as odorless white or yellowish-white crystals or a white or creamy-white crystalline powder. (NTP, 1992)
National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
CAMEO Chemicals
Testosterone propionate is a steroid ester.
ChEBI
Testosterone propionate is a slower-releasing anabolic steroid with a short half-life. It is a synthetic androstane steroid derivative of testosterone in the form of 17β propionate ester of testosterone. Testosterone propionate was developed initially by Watson labs, and FDA approved on February 5, 1974. Currently, this drug has been discontinued in humans, but the vet application is still available as an OTC.
DrugBank
View More… See also:  [image]Testosterone (has active moiety); Estradiol Benzoate; Testosterone Propionate (component of). 1 Structures
[image]Testosterone (has active moiety); Estradiol Benzoate; Testosterone Propionate (component of). 1 Structures
1.1 2D Structure
Structure Search
Get Image
Download Coordinates
Chemical Structure Depiction
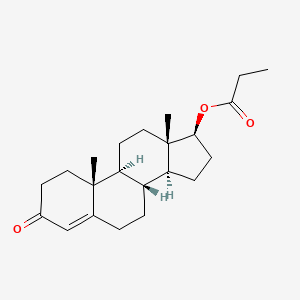 [image]
[image]
Full screenZoom inZoom out
PubChem 1.2 3D Conformer
PubChem 2 Names and Identifiers
2.1 Computed Descriptors
2.1.1 IUPAC Name
[(8R,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl] propanoate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
PubChem 2.1.2 InChI
InChI=1S/C22H32O3/c1-4-20(24)25-19-8-7-17-16-6-5-14-13-15(23)9-11-21(14,2)18(16)10-12-22(17,19)3/h13,16-19H,4-12H2,1-3H3/t16-,17-,18-,19-,21-,22-/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
PubChem 2.1.3 InChIKey
PDMMFKSKQVNJMI-BLQWBTBKSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
PubChem 2.1.4 SMILES
CCC(=O)O[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
PubChem 2.2 Molecular Formula
C22H32O3
Computed by PubChem 2.2 (PubChem release 2021.10.14)
CAMEO Chemicals; PubChem 2.3 Other Identifiers
2.3.1 CAS
57-85-2
Australian Industrial Chemicals Introduction Scheme (AICIS); CAMEO Chemicals; CAS Common Chemistry; ChemIDplus; DrugBank; DTP/NCI; EPA Chemicals under the TSCA; EPA DSSTox; European Chemicals Agency (ECHA); FDA Global Substance Registration System (GSRS); Human Metabolome Database (HMDB); New Zealand Environmental Protection Authority (EPA) 2.3.2 Deprecated CAS
1050678-75-5
ChemIDplus; EPA Chemicals under the TSCA 2.3.3 European Community (EC) Number
200-351-1
European Chemicals Agency (ECHA) 2.3.4 UNII
WI93Z9138A
FDA Global Substance Registration System (GSRS) 2.3.5 ChEBI ID
CHEBI:9466
ChEBI 2.3.6 ChEMBL ID
CHEMBL1170
ChEMBL 2.3.7 DrugBank ID
DB01420
DrugBank 2.3.8 DSSTox Substance ID
DTXSID9036515
EPA DSSTox 2.3.9 HMDB ID
HMDB0015489
Human Metabolome Database (HMDB) 2.3.10 KEGG ID
C08158
KEGG
D00959
KEGG 2.3.11 Lipid Maps ID (LM_ID)
LMST02020076
LIPID MAPS 2.3.12 Metabolomics Workbench ID
35355
Metabolomics Workbench 2.3.13 NCI Thesaurus Code
C863
NCI Thesaurus (NCIt)
C2298
NCI Thesaurus (NCIt) 2.3.14 Nikkaji Number
J4.584E
Japan Chemical Substance Dictionary (Nikkaji) 2.3.15 NSC Number
9166
DTP/NCI 2.3.16 PharmGKB ID
PA164751373
PharmGKB 2.3.17 Pharos Ligand ID
VVWPQAWTMKTW
Pharos 2.3.18 Wikidata
Q10354588
Wikidata 2.3.19 Wikipedia
Testosterone_propionate
Wikipedia 2.4 Synonyms
2.4.1 MeSH Entry Terms
- Agovirin
- Eifelfango, Testosteron Propionat
- Testosteron propionat Eifelfango
- Testosterone Propionate
- Virormone
Medical Subject Headings (MeSH) 2.4.2 Depositor-Supplied Synonyms
- testosterone propionate
- 57-85-2
- Agovirin
- Androlon
- Androteston
- Aquaviron
- Hormoteston
- Enarmon
- Bio-testiculina
- Androtest P
- Androsteston
- Masenate
- Orchistin
- Pantestin
- Propiokan
- Solvotest
- Sterandryl
- Synandrol
- Synerone
- Testaform
- Testodet
- Testodrin
- Testogen
- Testolets
- Testonique
- Testormol
- Testosid
- Testoxyl
- Uniteston
- Nasdol
- Orchiol
- Telipex
- Testrex
- Tostrin
- Vulvan
- Neo-Hombreol
- Okasa-Mascul
- Andrusol-P
- Oreton propionate
- Andronate
- Testex
- Orchisterone
- Testosteroni propionas
- Androgen
- Testosteron propionate
- Primotestone
- NSC 9166
- Homandren (amps)
- Testoviron (ampule)
- CCRIS 575
- Testosterone-17beta propionate
- Testosterone-17beta-propionate
- Testosterone, propionate
- Testosterone-17-beta-propionate
- Testosterone (propionate)
- Testosteron 17-propionate
- Testosterone-17-propionate
- NSC-9166
- Testosterone propionate ciii
- EINECS 200-351-1
- UNII-WI93Z9138A
- DTXSID9036515
- 17beta-(Propionyloxy)androst-4-en-3-one
- AI3-26378
- WI93Z9138A
- 17beta-Hydroxyandrost-4-en-3-one propionate
- NRB-03689
- 17beta-Hydroxy-4-androsten-3-one 17-propionate
- Androst-4-en-3-one, 17beta-hydroxy-, propionate
- delta(sup 4)-Androstene-17-beta-propionate-3-one
- CHEMBL1170
- 17beta-Hydroxyandrost-4-en-3-one-17beta-propionate
- CHEBI:9466
- DTXCID7016515
- NSC9166
- Testosterone 17.beta.-propionate
- Testosterone-17.beta.-propionate
- Testosterone propionate [USP:JAN]
- [(8R,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl] propanoate
- NCGC00016254-01
- Testosterone 17-propionate
- (17beta)-3-oxoandrost-4-en-17-yl propanoate
- (17beta)-3-oxoandrost-4-en-17-yl propionate
- 17-propionyl-17beta-hydroxyandrost-4-en-3-one
- Testosterone propionate (USP:JAN)
- TESTOSTERONE PROPIONATE (MART.)
- TESTOSTERONE PROPIONATE [MART.]
- CAS-57-85-2
- Androst-4-en-3-one, 17-(1-oxopropoxy)-, (17.beta.)-
- testosteron-17-propionat
- TESTOSTERONE PROPIONATE (EP MONOGRAPH)
- TESTOSTERONE PROPIONATE (USP IMPURITY)
- TESTOSTERONE PROPIONATE [EP MONOGRAPH]
zedCID]) - Testosterone propionate, solid
- SPBio_002261
- BPBio1_000356
- [GTPL7100](No items found - PubChem Substance - NCBI[StandardizedCID
- Androst-4-en-3-one, 17-(1-oxopropoxy)-(17-beta)-
- HMS1569A04